Assignments
Report on Economic and Energy Sector Analysis of Denmark
Vanadium Flow Batteries
The Vanadium Redox Battery (VRB), also known as the Vanadium Flow Battery (VFB) or Vanadium Redox Flow Battery (VRFB), is a type of rechargeable flow battery that store and release energy through the use of vanadium ions in a liquid electrolyte. Unlike traditional batteries, which rely on solid electrodes, VFBs use two separate liquid electrolyte solutions, typically vanadium-based, stored in external tanks. The energy is stored in the electrolytes, which are pumped through the battery's electrochemical cells during charging and discharging cycles.
Development of Vanadium Flow Batteries
The key development that led to the modern vanadium flow battery occurred in the 1980s. Dr. Maria Skyllas-Kazacos and her team at the University of New South Wales (UNSW) in Australia made a groundbreaking discovery: vanadium could be used as the central element in a flow battery, owing to its ability to exist in multiple oxidation states. In 1986 Dr. Skyllas-Kazacos and her research group published their seminal paper on the use of vanadium for redox flow batteries. They identified that vanadium could exist in four oxidation states (+2, +3, +4, and +5), which would enable both the positive and negative electrolytes to be made of vanadium solutions without risk of cross-contamination between the two electrolytes. This solved a critical challenge faced by previous flow battery technologies. The ability of vanadium to exist in multiple oxidation states (unlike other metals like zinc or iron, which only have two states) enabled a much more stable and efficient energy storage system.
Working Principle
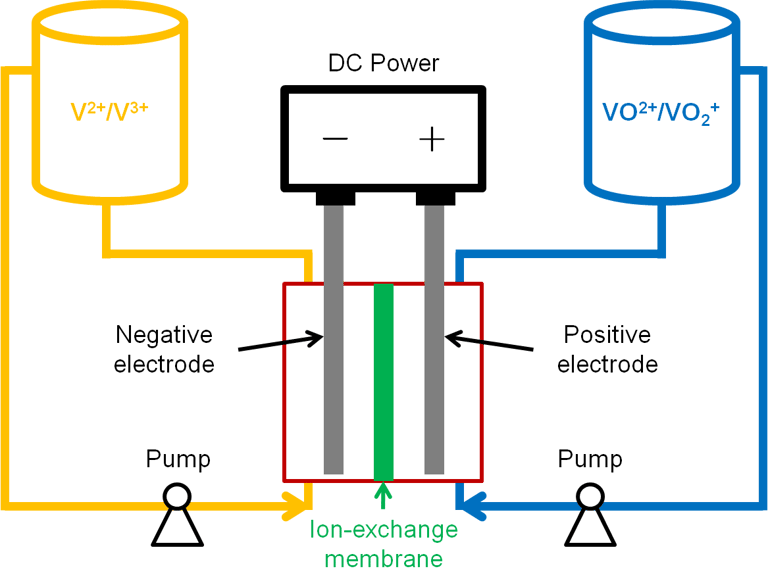
Schematic of a vanadium redox-flow battery.
Vanadium redox-flow battery has two chambers, a positive chamber and a negative chamber, separated by an ion-exchange membrane. These two chambers are circulated with electrolytes containing active species of vanadium in different valence states, VO2+/VO2+ in the positive electrolyte and V2+/V3+ in the negative electrolyte.
During discharge process, VO2+ is reduced to VO2+ at the positive electrode and V2+ is oxidized to V3+ at the negative electrode, as shown in Equation (1) and (2). The reactions proceed in the opposite direction during charge process. The active species are normally dissolved in a strong acid, and the protons transport across the ion-exchange membrane to balance the charge. The standard voltage produced by the vanadium redox-flow battery system is 1.25 V. [1-3]
Positive Electrode: VO2+ + H2O - e- → VO2+ + 2 H (1)
Negative Electrode: V3+ + e- → V2+ (2)
Applications of Vanadium Redox-Flow Batteries
Vanadium Flow Batteries (VFBs) are ideal for large-scale energy storage and grid stabilization due to their scalability and long cycle life. Here are key applications:
Grid Energy Storage: VFBs balance supply and demand by storing excess energy during low-demand periods and releasing it during peak demand, helping to stabilize the grid and integrate renewable energy sources like solar and wind.
Renewable Energy Storage: VFBs store intermittent renewable energy, such as solar and wind, for use when generation is low, ensuring a continuous power supply.
3. EV Charging Stations: VFBs support EV charging stations by storing energy for consistent charging and reducing grid demand during peak hours.
4. Backup Power: VFBs provide backup power for critical infrastructure (e.g., hospitals, data centers) and essential services in case of grid outages or natural disasters.
5. Residential Solar Storage: Homeowners can store excess solar energy in VFBs for use during cloudy days or at night, improving the efficiency of solar power systems.
Advantages of Vanadium Redox- Flow Batteries
Long Cycle Life: Vanadium Flow Batteries have an exceptionally long cycle life, typically exceeding 10,000 cycles. This is due to the fact that the electrodes do not degrade during the charge/discharge process, as the electrolyte is stored externally.
Scalability: VFBs can be easily scaled for larger applications by increasing the size of the electrolyte tanks or adding more cell stacks. This makes them ideal for large-scale energy storage applications like grid storage or renewable energy integration.
Safety: Unlike lithium-ion batteries, which can be prone to overheating or fires, VFBs use aqueous electrolytes, making them non-flammable and safer to operate, especially in large-scale installations.
Environmental Sustainability: Vanadium is a relatively abundant metal and the electrolyte used in VFBs is recyclable. Additionally, VFBs do not contain hazardous materials like heavy metals (e.g., lead or cadmium), making them a more environmentally friendly option.
High Efficiency: VFBs typically operate at high round-trip efficiencies (70-80%), meaning they can store and release a large portion of the energy they receive without significant losses.
Independent Power and Energy Scaling: The power capacity (determined by the number of cells) and energy capacity (determined by the size of the electrolyte storage) can be independently scaled. This flexibility allows for optimization based on the application requirements.
Stable Performance: Due to their stable chemistry and design, VFBs provide consistent performance over a long lifespan, without significant capacity degradation over time, unlike some other battery chemistries.
Disadvantages of Vanadium Redox-Flow Batteries
1. High Initial Cost: One of the most significant drawbacks of VFBs is their high upfront cost. The materials used, such as vanadium and specialized membranes, are expensive. Additionally, the complex systems, including pumps and storage tanks, contribute to the high capital expense.
2.Lower Energy Density: VFBs have a relatively low energy density compared to other battery technologies, such as lithium-ion batteries. This means they require larger volumes and more space for the same amount of energy storage, making them less suitable for portable or space- constrained applications.
3. Complexity of System: VFBs require a more complex system with pumps, pipes, and electrolyte storage tanks, which increases the operational complexity and the need for ongoing maintenance. This complexity can lead to higher maintenance costs over time.
4. Temperature Sensitivity: Vanadium Flow Batteries can be sensitive to extreme temperatures, which could impact their performance. Temperature control mechanisms may be needed to maintain optimal operation in harsh environments, adding to the cost and complexity.
Current Research and Developments
Vanadium Recovery and Recycling: A key area of research is the development of efficient methods to recover and recycle vanadium from spent batteries, which would help reduce the environmental impact and cost of raw materials.
Optimization of Electrolyte Chemistry: Researchers are investigating ways to improve the electrolyte chemistry to increase the efficiency and energy density of VFBs. This includes exploring different vanadium-based compounds and additives to improve performance.
Cost Reduction: Ongoing research focuses on reducing the overall cost of VFBs by identifying alternative materials, improving manufacturing processes, and developing cheaper electrode and membrane technologies.
Solid-State Vanadium Flow Batteries: Some researchers are exploring the potential of solid-state VFBs, which could offer higher energy density, lower weight, and more compact designs than traditional liquid-based systems.
Conclusion
Vanadium Flow Batteries offer an innovative and promising solution for large-scale energy storage, with their ability to scale efficiently, provide long cycle life, and operate safely. Despite the challenges of high initial costs and lower energy density, ongoing research and development efforts are likely to lead to further improvements in performance, cost reduction, and scalability. With advancements in materials and system design, VFBs could play a crucial role in the future of renewable energy storage, grid stabilization, and off-grid applications.